iDrug: Advanced Drug Interaction
Overview
iDrug represents a cutting-edge and professional SDK, UI and database designed to provide comprehensive insights into various aspects of drug interactions, including:
- Drug-Drug Interactions (DDIs): Information on potential adverse reactions and compatibility between medications.
- Drug-Food Interactions (DFIs): Analysis of how dietary components affect drug efficacy and safety.
- Drug-Disease Similarity Interactions (DDSIs): Identification of therapeutic duplications and contraindications based on disease profiles.
- Therapeutic Duplications: Detection and management of overlapping medications within treatment plans.
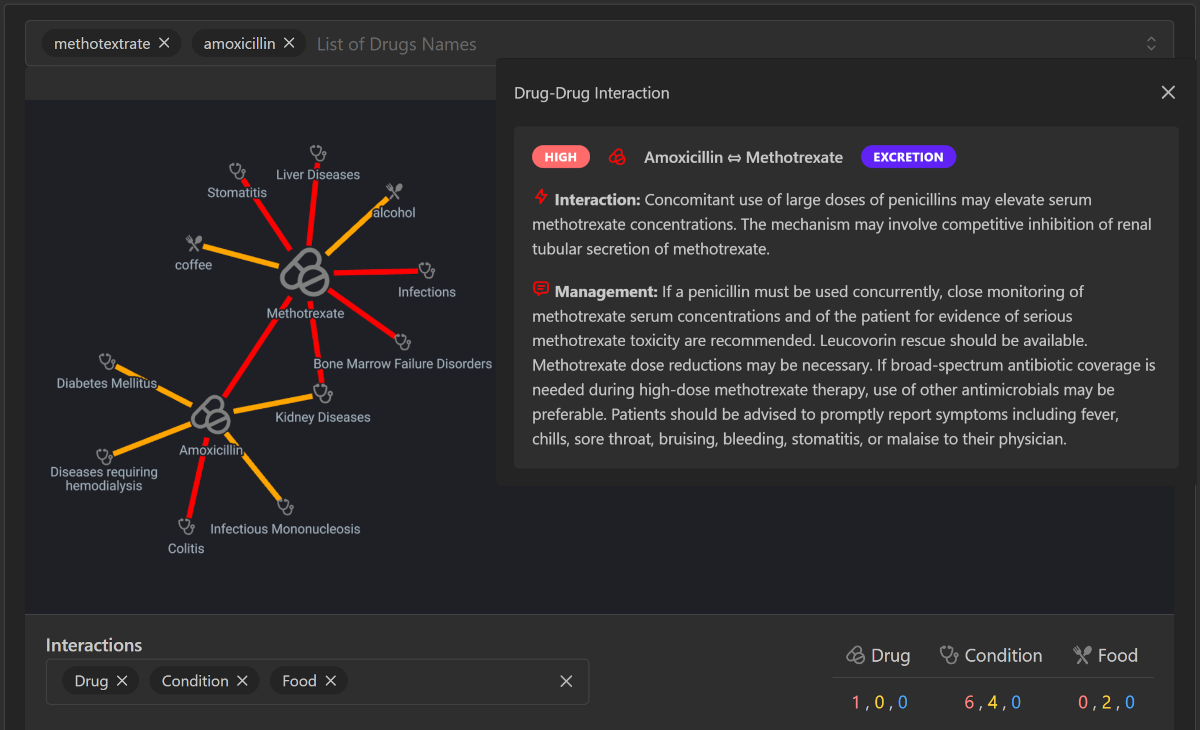
Expanded Features
The latest edition of iDrug introduces an array of advanced features, broadening the scope of drug interaction analysis:
- Mechanism Descriptions: Detailed annotations on the biochemical or pharmacokinetic mechanisms behind interactions.
- Risk Levels: Categorization of interactions based on severity and likelihood of adverse effects.
- Management Strategies: Suggested solutions and recommendations for mitigating risks associated with interactions.
- Updated ATC Codes: Integration of updated Anatomical Therapeutic Chemical (ATC) classification codes for newly introduced and existing medications.
Tools and Functionalities
iDrug offers specialized tools to streamline the analysis, visualization, and integration of drug interaction data:
- User Interface Design: Resources to construct intuitive and efficient drug interaction functionalities tailored for healthcare providers and researchers.
- Knowledge Graph Representations: Advanced graph-based models to visualize and explore complex interaction networks.
- Comprehensive Drug/Food/Disease Interaction Database: A unified repository with detailed insights into DDIs, DFIs, and DDSIs.
Integration and Classification
iDrug seamlessly integrates with external standards and resources to enhance utility and accessibility:
- HPRA Medication Synchronization: Incorporates synchronization capabilities with HPRA (Health Products Regulatory Authority) database for real-time updates on medications.
- WHO Anatomical Therapeutic Chemical (ATC) Classification: Full compatibility with global WHO standards for drug categorization and classification.
External Resources
To further enrich data analysis and understanding, iDrug connects with additional resources, including:
- Wikipedia: General drug-related information and public domain insights.
- ChEMBL: Bioactivity data for drug discovery and research.
- PubChem: Comprehensive molecular and chemical information.
Applications
The iDrug database is an invaluable tool for:
- Healthcare Professionals: Ensuring safe prescribing practices by identifying and managing potential risks.
- Pharmacists: Streamlining medication reviews and optimizing patient care.
- Researchers: Advancing drug interaction studies with high-quality data and visualization tools.
With its robust features, expanded data annotations, and seamless integration capabilities, iDrug stands as a vital asset for the medical and pharmaceutical community.